Homework 4, due April 6, 2004
Chapter
4, problem 13 (5 points): The labeled transitions below represent an electron moving between
energy levels in hydrogen. Answer each of the following questions and explain
your answers.
a)
Which
transition could represent an electron that gains 10.2 eV of energy?
Transition B – the electron absorbs a photon and is
able to jump to a level with higher energy, the energy difference between the
levels is 10.2 eV
b)
Which
transition represents an electron that loses 10.2 eV of energy?
Transition C – the electron jumps from the 10.2 eV
level down to the 0.0 eV level and has to get rid of the extra energy – it
sends off a photon with an energy equal to the difference of 10.2 eV
c)
Which
transition represents an electron that is breaking free of the atom?
Transition E – the electron gains so much energy
that it can leave the atom, left is a free electron and an ionized atom
d)
Which
transition, as shown, is not possible?
Transition D – You cannot have an electron in
between the discrete orbits.
e)
Describe
the process taking place in transition A.
The electron can jump more than one level at a time,
as long as energy is conserved. In this case, it has to send off a photon with
12.1 eV to be able to go directly to the ground state.
Chapter
6, problem 11 (5 points): Suppose the surface temperature of the Sun were about 12,000 K,
rather than 6,000 K.
a)
How
much more thermal radiation would the Sun emit?
Stefan-Boltzman law: L ~ R2T4 ® L2 ~ R2T24
= R2*(2*T1)4 = 24*R2T4
= 16*L1
® it would emit 16 times more
thermal radiation
b)
How
would the thermal radiation spectrum of the Sun be different?
Wien’s law: lpeak ~ 1/T ® a hotter star has a lower
peak wavelength and is brighter ® spectrum would be shifted
towards the blue
®
lpeak = 2,900,000/12,000 = 242 nm
which is in the UV
c)
Do
you think it would still be possible to have life on Earth? Explain.
Life would probably still be possible, but more
difficult. The increase in UV radiation would affect our atmosphere. The Earth
will get hotter as well, since the Sun would put out more total radiation.
Chapter
12, problem 9 (5 points): The total mass of the Sun is about 2x1030 kg, of which
about 75% was hydrogen when the Sun formed. However, only about 13% of this
hydrogen ever becomes available for fusion in the core; the rest remains in
layers of the Sun where the temperature is too low for fusion.
a)
Based
on the given information, calculate the total mass of hydrogen available for
fusion over the lifetime of the Sun.
b)
The
only hydrogen available are the 13% of the total amount of hydrogen which is in
the core. The total amount of hydrogen is 75% of the total mass.
® 13% of 75% of 2x1030 kg = 0.13 * 0.75 * 2x1030 kg =
1.95x1029 kg
c)
Combine your results from part (a) and the fact that the Sun fuses about 600
billion kg of hydrogen each second to calculate how long the Sun’s initial
supply of hydrogen can last. Give your answer in both seconds and years.
600 billion kg = 1.95x1029
kg ® x = 1.95x1029
kg * 1sec = 3.25x1017 seconds
1 sec x 600x109
kg
1 year = 31,536,000 sec ® x = 1.03x1010 years = 10.3
billion years
The Sun can burn hydrogen for 10
billion years.
c)
Given that our solar system is now about 4.6 billion years old, when will we
need to worry about the Sun running out of hydrogen for fusion?
10.3 billion – 4.6 billion = 5.7 billion years ® We should start worrying about that in about
5.4 billion years, if we are still here than.
Chapter
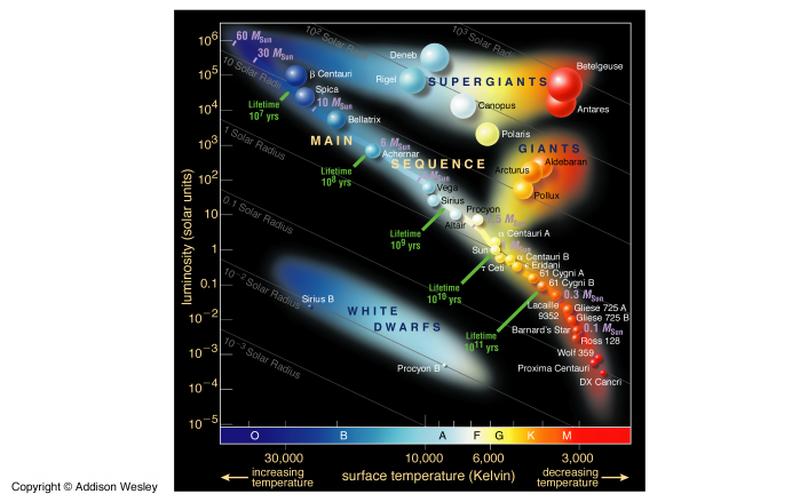
13, problem 12 (5 points): Draw a sketch of a basic Hertzsprung-Russel diagram (H-R diagram).
Label the main sequence, giants, supergiants, and white dwarfs. Where on this
diagram do we find stars that are cool and dim? Cool and luminous? Hot and dim?
Hot and bright?
Cool and dim: lower right corner
(low temperature, low luminosity)
Cool and luminous: upper right
corner (low temperature, high luminosity)
Hot and dim: lower left corner (high
temperature, low luminosity)
Hot and bright: upper left corner
(high temperature, high luminosity)